The effect of SEBS-g-MA compatibilizer on properties of polymer compounds

The effect of SEBS-g-MA compatibilizer physical and mechanical properties of polymer compounds
Styrene-Ethylene-Butylene-Styrene (SEBS) is one type of different types of Styrene thermoplastic elastomers. In chemical structure of SEBS terpolymer, the terminal blocks are styrene (thermoplastic) and the middle blocks are ethylene-butylene (elastic) (figure 1). This new thermoplastic elastomer is very resistant to environmental conditions such as temperature, UV radiation and mechanical wear. The lack of double bonds in this thermoplastic elastomer makes it suitable for use in cases where styrene butadiene styrene cannot be used. SEBS is a thermoplastic elastomer which that has the properties of an elastomer along with the low cost of the thermoplastic process at the same time. Excellent wear resistance in SEBS-based mixtures is due to the absence of double chains in their polymer structure.
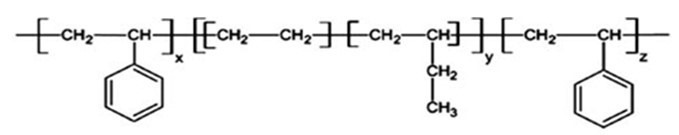 Fig 1. Chemical structure of SEBS
Fig 1. Chemical structure of SEBS
Due to the flexibility in the formulation, it is possible to prepare products in a wide range of hardness for different applications from SEBS in the industry. The multitude properties of these materials have led to the expansion of global markets for these materials, including in various tools and accessories (figure 2).
 Fig 2. Application of SEBS in various tools
Fig 2. Application of SEBS in various tools
The most remarkable features of this polymer are: wide range of hardness and excellent resistance to aging, wide range of colorability, very good processability at low temperatures and resistance to high temperatures. Besides, this polymer as a thermoplastic elastomer has high impact resistant and it is used as an impact modifier in some compounds. To process SEBS, like any other polymers, different additives is used. These additives during the process, or immediately after polymerization are added to the system to prevent possible defects during the process of parts and during use. These additives are used in several categories of antioxidants, light stabilizers, fillers, reinforcements and softeners. Antioxidants are mostly used in the production of polyolefins and styrene-based compounds. In addition, because the elastic modulus and hardness of SEBS is low, mineral reinforcements such as glass fibers, talc, calcium carbonate, etc. are used in it. But SEBS are not compatible with most mineral fillers and reinforcing materials such as glass fibers, talc, calcium carbonate, etc., because they are different from each other in terms of polarity, and there is a need for the presence of polymer compatibilizers.
Compatibilizers or coupling agents reduce the mixing enthalpy and interfacial free energy. Also, they are chemically similar to both phases and thermodynamically compatible with one or both phases. In many applications, SEBS grafted with maleic anhydride has been used as a compatibilizer. The chemical structure of SEBS grafted by maleic anhydride is shown in Figure 3.
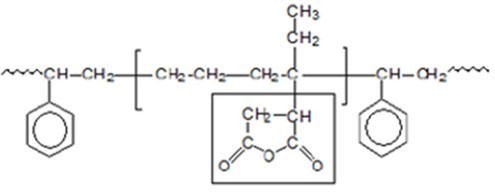 Fig 3. Chemical structure of SEBS grafted by maleic anhydride
Fig 3. Chemical structure of SEBS grafted by maleic anhydride
The function of this compatibilizer is that it is connected to minerals from the side of the maleic anhydride molecule with high polarity and interacts with the polymer in the compound from the side of its polymer chain, and in this way, the connection between the mineral particles and the polymer is established.
In the rest following, we will examine the compatibilizing effect of maleic SEBS on the properties of compounds containing SEBS and calcium carbonate, as well as on the properties of compounds containing polypropylene and polyamide.
In the first stage, compounds with the same polymer base as the coupling agent were tested, and the raw materials used included styrene-ethylene-butylene-styrene terpolymer (SEBS), maleic anhydride, calcium carbonate modified with stearic acid and other additives. To prepare the above compounds, first, the coupling agent SEBS-g-MA was prepared by an internal mixer, and then 6 compounds including SEBS components, calcium carbonate, SEBS-g-MA and other additives were prepared in a twin screw extruder. The names and formulas of these compounds are listed in Table 1.
Table 1. Names and formulas of prepared compounds
| Name | SEBS/ PHR | CaCO3/ PHR | SEBS-g-MA/PHR |
| SN0 | 100 | 0 | 0 |
| SN1 | 100 | 3 | 0 |
| SN2 | 100 | 5 | 0 |
| SN3 | 100 | 3 | 1.5 |
| SN4 | 100 | 5 | 1.5 |
| SN5 | 100 | 3 | 3 |
| SN6 | 100 | 5 | 3 |
The compounds prepared for 3 minutes at a temperature of 170 degrees Celsius and a pressure of about 200 bar were molded in the form of a sheet under a hot press, and after making standard samples and preparing dumbbell-shaped samples from the obtained compounds, tensile tests were performed according to the ASTM D638 standard and hardness according to ASTM D2240 standard were performed to obtain the properties of the above compounds. The results of tensile and hardness tests for the prepared compounds are given in Table 2.
Table 2. Amounts of hardness (Shore A), tensile strength, and elongation at break
| Hardness/Shore A | Tensile strength/Mpa | Elongation at break (%) | Compound |
| 65 | 4 | 370 | SN0 |
| 69 | 4.4 | 319 | SN1 |
| 70 | 4.5 | 296 | SN2 |
| 71 | 5.3 | 267 | SN3 |
| 72 | 5.1 | 254 | SN4 |
| 75 | 4.8 | 241 | SN5 |
| 76 | 4.5 | 229 | SN6 |
Tensile strength values in Table 2 indicate that with the addition of calcium carbonate, the tensile strength increases to a small amount, but with the addition of the SEBS-g-MA coupling agent, the increase in tensile strength will be higher as follows that by adding 1.5 phr of SEBS-g-MA coupling agent to the compound containing 3% calcium carbonate, the tensile strength increases by 32.5% and this increase is due to two reasons: Firstly, a strong chemical bond is established between the polar group of stearic acid and calcium carbonate, and calcium stearate is formed, which improves the compatibility between the polymer matrix and calcium carbonate particles. Secondly, the SEBS-g-MA coupling agent, due to the presence of the polar carbonyl group in maleic anhydride, has the possibility of establishing a hydrogen bond with the ester group of the stearate coating on the surface of calcium carbonate, and its polymer chains are mixed with the SEBS matrix from the other side. In one hand, the strength and resistance of the above compounds depends on the amount of adhesion between the reinforcing particles and the polymer. On the other hand, by increasing the coupling agent to 3 PHR, the tensile strength decreases compared to 1.5 PHR, and the reason is that by increasing the compatibilizer percentage and since the compatibilizer is considered a hard component compared to the polymer, as a result, it declines flexibility and tensile strength.
Elongation at break percentage in the case of prepared compounds is reduced compared to SN0 control sample, because on the one hand, calcium carbonate mineral particles can act as stress concentration points or initiate cracks in the samples and as a result reduce the elongation to breaking point and on the other hand, the reduction of the elongation at the breaking point can be attributed to the plastic nature of SEBS-g-MA because it has less elastomeric properties than SEBS.
By adding the SEBS-g-MA coupling agent to the amount of 3 PHR in the compound containing 5% calcium carbonate, the hardness increases up to 17%, and in general, the hardness of all samples increases due to the addition of the coupling agent SEBS-g-MA to SEBS. In order to explain this phenomenon, it should be pointed out that the dispersed particles of calcium carbonate act as hard particles and prevent the penetration of the needle into the sample. If the particles are not completely distributed and remain in the polymer in the form of clusters, they act like a two-phase system, so that these particles act as a hard phase and the polymer acts as a soft phase, so finally the hardness of the system decreases, but due to the uniform distribution of calcium carbonate particles due to the presence of SEBS-g-MA within the polymer system, the indentation resistance increases. In the second step, the effect of SEBS-g-MA coupling agent on the properties of polypropylene/polyamide compounds was investigated, and for this purpose, SEBS-g-MA agent was added in amounts of 5 and 10% to compounds containing 80% polypropylene and 20% polyamide, and compounds containing the opposite ratio, i.e. 20% polypropylene and 80% polyamide, were added, and the impact properties of the above compounds were measured by the izod impact test according to ASTM D256, the results are shown in Table 3.
Table 3. Impact strength amounts of polypropylene/polyamide compounds
| Item | Izod impact strength (kJ/m2) |
| PA/PP (80:20) without compatibilizer | 4 |
| PA/PP (80:20) with 5% SEBS-g-MA | 15.1 |
| PA/PP (80:20) with 10% SEBS-g-MA | 29.5 |
| PA/PP (20:80) without compatibilizer | 2.2 |
| PA/PP (20:80) with 5% SEBS-g-MA | 6.3 |
| PA/PP (20:80) with 10% SEBS-g-MA | 12.2 |
From the data in Table 3, it is clear that the impact resistance increases with the addition of SEBS-g-MA agent to polypropylene/polyamide compounds, and when 10% is added, the impact resistance increases about 6 folds. This is for two reasons: Firstly, SEBS-g-MA is used to increase impact properties due to its elastomeric nature, and secondly, SEBS-g-MA acts as a binding agent between polypropylene and polyamide and makes the compound homogenous. The mechanism can be seen in figure 4.
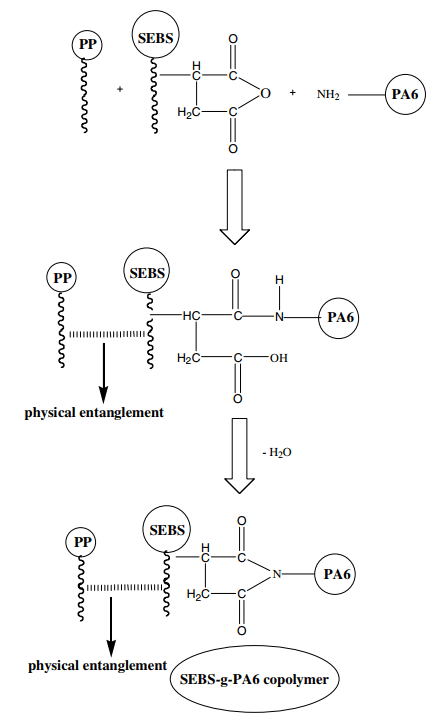 Fig 4. Mechanism of effect of SEBS-g-MA agent on polypropylene and polyamide bonding
Fig 4. Mechanism of effect of SEBS-g-MA agent on polypropylene and polyamide bonding
In another case, the effect of SEBS-g-MA coupling agent on the properties of polyamide 6 and polyamide 66 compounds with glass fibers was investigated, the results of which are shown in Tables 4 and 5:
Table 4. Effect of adding 5% SEBS-g-MA on the impact of Polyamide6/glass fibers compound
| Item | PA6/GF | PA6/GF/5% SEBS-g-MA |
| Tensile strength/ MPa | 138 | 135 |
| Elongation at break (%) | 2.1 | 2.6 |
| Izod Impact, Notched/ KJ/m2 | 9 | 27.5 |
Table 5. Effect of adding 5 and 10% SEBS-g-MA on PA6/GF compound
| Item | PA66/GF | PA66/GF/5% SEBS-g-MA | PA66/GF/10% SEBS-g-MA |
| Tensile strength/ MPa | 162 | 117 | 98 |
| Notch impact strength/ KJ/m2 | 11 | 22 | 30 |
From the data in Tables 4 and 5, it is clear that the impact resistance property increases with the addition of SEBS-g-MA agent to polyamide/glass fiber composites, and in the case of polyamide 6, by adding 5% of SEBS-g-MA to the impact resistance of compound increases about 3 times, and this increase in the case of polyamide 66 is achieved by adding 10% of SEBS-g-MA agent, and the reason for the improved impact property can be attributed to the elastomeric nature of SEBS-g-MA agent and its compatibility with polyamide and glass fibers.
Author: Emad Izadi Vasafi